Ship Corrosion & Strategies for Prevention
Ship Corrosion
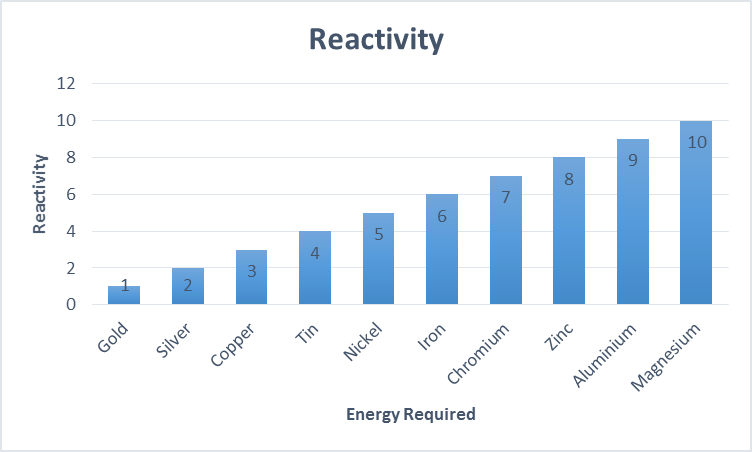
Ship corrosion is a major hazard for the industry. The deterioration of these structures causes higher maintenance costs, early system failures, or an overall shortened service life. Corrosion is a natural process which as a result, reduces metallic elements back to their original state. All metals have different levels of risk for example gold is the least corrosive while magnesium is the most corrosive.
Metals with more energy also corrode more easily.
5 Types of Ship Corrosion
1.) Galvanic Corrosion
Galvanic corrosion is the most common form of corrosion and therefore the most dangerous. This occurs when two or more metals are in contact with each other, while submerged in an electrolyte solution like saltwater. As a result, the more reactive metal begins to deteriorate at a faster pace which is never a good sign in regards to personal safety. Physical signs of corrosion are flaking and blistering of paint and eventually lead to pitting of the metal.
2.) Stress Corrosion Cracking (SCC)
So, Stress corrosion cracking leads to rapid failure of ductile alloys. In addition, the stainless steel develops microscopic cracks from tensile stress and forcing the object in opposite directions.
3.) Crevice Corrosion
Therefore, Crevice corrosion involves the interaction of one metal part with two connected environments such as a tight, confined space, where elements like oxygen are limited. In conclusion, depending on the environments involved, pitting or cracking may occur, causing some serious damage.
4.) Inter-granular Corrosion (IGC)
Hence, Inter-granular attack is the differences between granular boundaries and granular bodies of a metal which occurs at the microscopic level. The surface area crystallizes a metal and is more vulnerable to corrosion than their lower layers causing inter-granular fractures along the metals surface.
5.) Flow-Accelerated Corrosion (FAC)
Flow-accelerated corrosion this type of ship corrosion occurs from the constant flow of water against a metals surface which can be particularly harmful and may rapidly breakdown a vessels protected layers.
When a metal is affected by corrosion, an oxide layer is formed which in turn protects a metals lower layers from further harm. Removing oxidation begins when salt water flows over this layer.
Strategies for Prevention
Preventive measures will decrease maintenance costs, early system failures, and an overall improve service life of ships granted they are detected in enough time.
a. Modifying the Corrosive Environment
Hence, Inhibitors are salt water or electrolyte solutions which are controlled. Anodic inhibitors migrate to the anode creating a protective barrier which as a result, stops corrosion in its tracks. Therefore, Cathodic Inhibitors migrate to the cathode which prevents the absorption of oxygen or hydrogen.
In addition, Cathodic protection uses sacrificial metal to act as the anode which creates a protective barrier. This metal deteriorates first instead of the ships protected layers.
For example on the hull of a ship, zinc plates prevent corrosion of any sub-surfaces.
b. Protecting the Corrosive Environment
As a result, in the hope of protecting the vessel coat it with three different paint solutions.
Binders – Therefore, the binder is a film forming component of paint.
Pigments/Extenders – these powders mix with the binder at various particle sizes and enhance the overall effectiveness of the binders.
Solvents -Eventually evaporate from the surface and help for easy application.
In conclusion, it is important to have the proper plating before the equipment is put to work. As a result the equipment will last longer and will become resistant to the elements. Therefore American Plating Company is the best place to plate any equipment, we know exactly how to treat each metal for a flawless finish. In addition, we also know how to restore any metal to its former glory. So, bring those old parts or new parts down to us or contact us below we process jobs from all over the country! Lastly, we have been in business since 1944, and will give your business the old school professional touch.
Additional Information:
American Plating Welcomes Your Inquiries and Feedback!
blog or contact American Plating Company at (314) 776-0542
References:
Anderson, Colin. “Protection of Ships.” Newcastle University. WEB. 1 Sept. 2015.
Kulkarni, Mandar. “Corrosion in Marine Environment.” Academia. 2015. WEB. 1 Sept. 2015.