Stopping Corrosion
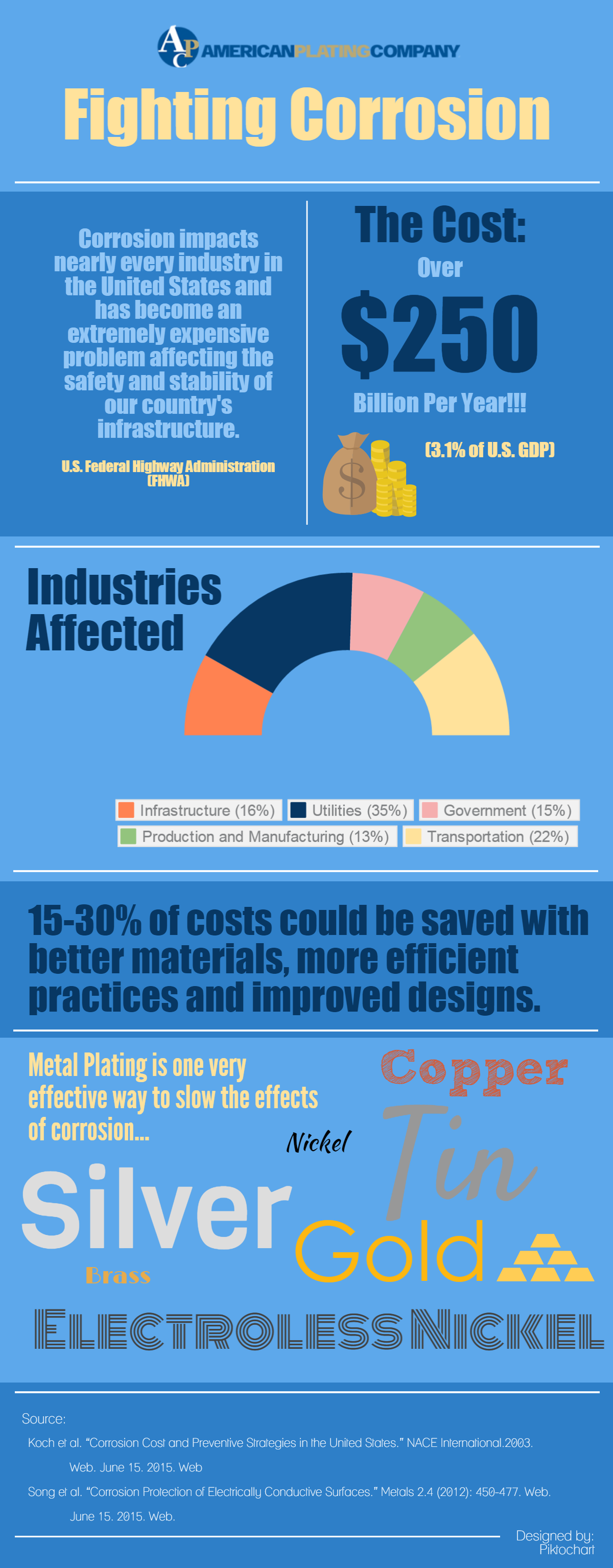
The Problem: Metallic corrosion impacts nearly every industry in the United States. As a result, based on a study conducted by the Federal Highway Administration (FHWA), corrosion causes over $250 billion in annual replacement and repair costs.
As a result this staggering expense is about 3% of the total US GDP. Unfortunately, corrosion is a natural ongoing occurrence that is not avoidable. However, there are ways to slow corrosive progression.
As a matter of fact, we cut 15-30% of costs by implementing new strategies with better materials, more efficient practices and better designs.
Is there a solution?
One effective way of stopping corrosion is to coat sensitive surfaces with a layering of a less reactive metal. However, not all metals are equal and some may provide more protection than others.
The Anodic Index can be a great tool for determining what metal may be best for any particular project.
Keep in mind: Exposing metals to different environments plays a major factor in deciding plating .
Harsh environments: Including hot, humid, or salty outdoor environments may cause rapid deterioration, a .15V or less difference on the Anodic Index is needed to prevent corrosion.
Normal environments: Including indoor facilities such as a warehouse which provide some protection from the elements, a .25V or less difference on the Anodic Index is needed.
Controlled environments: Including facilities with a controlled temperature, a .50V or less difference on the Anodic Index is needed.
Based on this information you may want to reference a galvanic corrosion chart such as the one below in order to finalize your decision.
The Anodic Index
|
Metallurgical Category |
Index (V) |
| Gold, solid & plated, Gold-platinum alloy | 0.00 |
| Rhodium plated on silver-plated copper | 0.05 |
| Silver, solid or plated; monel metal. High nickel-copper alloys | 0.15 |
| Nickel, solid or plated, titanium an s alloys, Monel | 0.30 |
| Copper, solid or plated; low brasses or bronzes; silver solder; German silvery high copper-nickel alloys; nickel-chromium alloys | 0.35 |
| Brass and bronzes | 0.40 |
| High brasses and bronzes | 0.45 |
| 18% chromium type corrosion-resistant steels | 0.50 |
| Chromium plated; tin plated; 12% chromium type corrosion-resistant steels | 0.60 |
| Tin-plate; tin-lead solder | 0.65 |
| Lead, solid or plated; high lead alloys | 0.70 |
| Aluminum, wrought alloys of the 2000 Series | 0.75 |
| Iron, wrought, gray or malleable, plain carbon and low alloy steels | 0.85 |
| Aluminum, wrought alloys other than 2000 Series aluminum, cast alloys of the silicon type | 0.90 |
| Aluminum, cast alloys other than silicon type, cadmium, plated and chromate | 0.95 |
| Hot-dip-zinc plate; galvanized steel | 1.20 |
| Zinc, wrought; zinc-base die-casting alloys; zinc plated | 1.25 |
| Magnesium & magnesium-base alloys, cast or wrought | 1.75 |
| Beryllium | 1.85 |
Gold
Gold is a noble metals, as a result, meaning it is resistant to corrosion and oxidation in most environments. It is the most non-reactive of all metals. Gold never reacts with oxygen, making this material resistant to rust or tarnishing. Perfectly applying a thin gold coating perfectly is rather important, as a result even the smallest holes will cause corrosion to occur. Gold is also an excellent conductor of electricity. If you have the money, gold would be your best defense for stopping corrosion.
Silver
Silver is also very close to a noble metal, however, sulfur may cause pitting and deterioration on the metals surface. It is also the most electrically conductive of all metals and is often used to coat semiconductors and other electrical devices. Like gold, silver is costly, making the metal less economical for many projects.
Tin & Nickel
Tin plating and nickel plating are not considered noble metals as a result; however, they are still very resistant to corrosion. These metals are referred to as passive metals and therefore, obtain their resistance to corrosion from a thin oxide film on the surface of the metal. The film inhibits corrosion and protects from further deterioration. However, it is important to note that both tin and nickel may be vulnerable to open pores on the plating depending on the density of the layering.
Electroless Nickel
Electroless Nickel is another excellent way to protect metals from corrosion. Because a current is not used, electroless nickel is evenly distributed on the surface and lacks the pours which may occur in the electrolytic nickel process. In addition, because electroless nickel contains phosphorus, the coating is even more resistant to corrosion.
Stopping Corrosion
References:
Koch et al. “Corrosion Cost and Preventive Strategies in the United States.” NACE International.2003.
Web. June 15. 2015. Web
Song et al. “Corrosion Protection of Electrically Conductive Surfaces.” Metals 2.4 (2012): 450-477. Web.
June 15. 2015. Web.